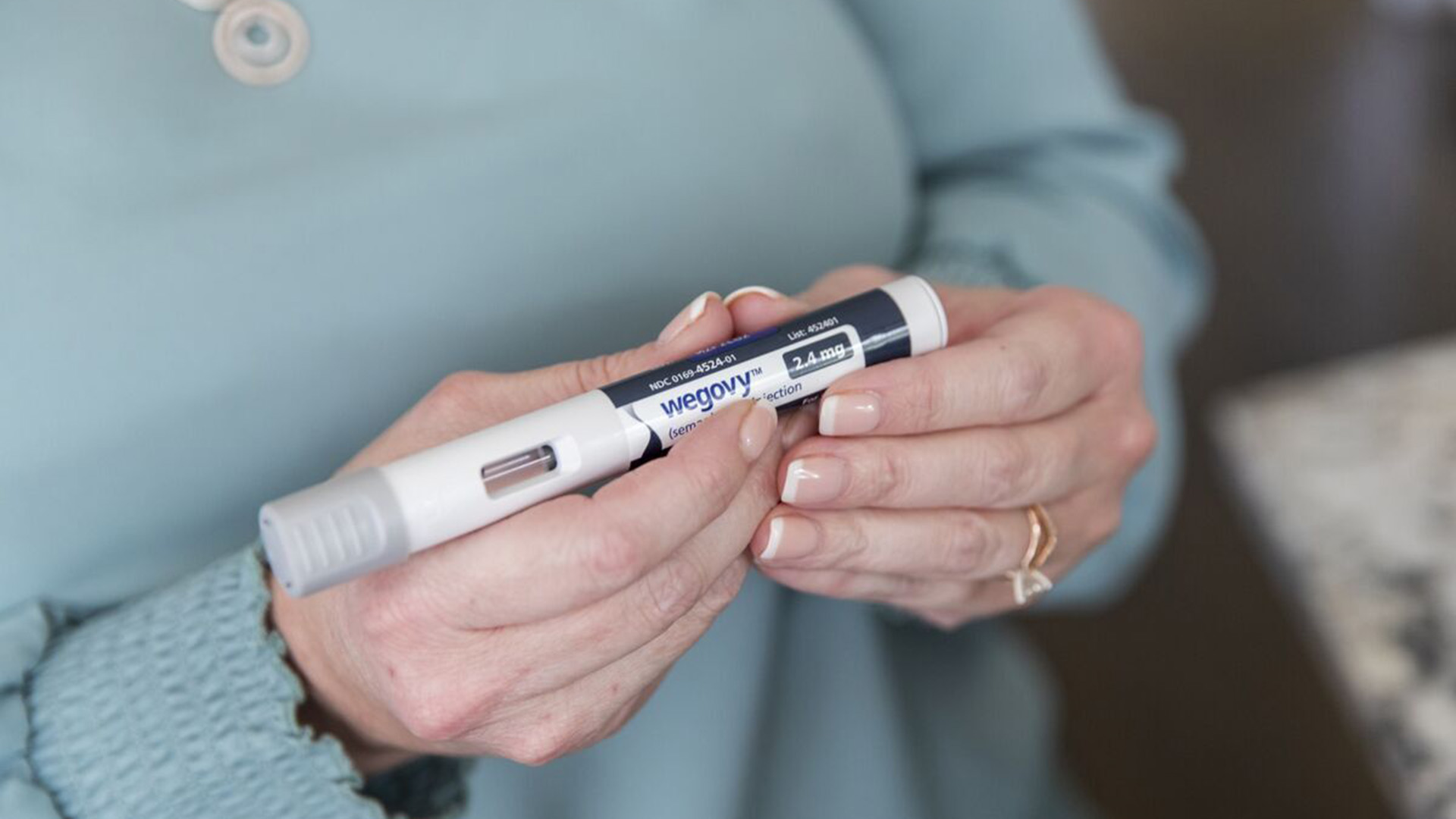
A recent trial revealed that Wegovy, a novel obesity medication, has significantly reduced the risk of severe heart problems by 20%, as reported by the drug’s manufacturer on Tuesday. This groundbreaking discovery challenges the prevailing notion that weight loss drugs primarily serve cosmetic purposes, potentially compelling insurers to reconsider their coverage policies.
This trial marks a pivotal moment as it is the first to showcase the enduring cardiovascular benefits of this new class of obesity drugs for individuals who are overweight but not diabetic.
Given that obesity affects approximately 100 million adults in the United States and contributes to nearly $150 billion in annual healthcare expenditures, these innovative treatments could play a crucial role in addressing some of the most substantial and expensive health challenges in American medicine.
The outcomes of the trial underscore the pressing necessity for individuals grappling with obesity to have access to this effective and safe medication to proactively prevent future diseases, emphasized Simon Cork, an obesity expert at Anglia Ruskin University in England, who is unaffiliated with the drug.
Tuesday’s announcement by Wegovy’s manufacturer, Novo Nordisk, lacked specific details, leaving many aspects undisclosed. While the company stated that the drug reduced the overall risk of heart attacks, strokes, and cardiovascular deaths by 20%, it did not provide a breakdown of the drug’s impact on each outcome separately.
Crucial information, such as the amount of weight lost by patients and the specifics of any side effects, along with data on how many patients discontinued the drug, was also missing. The data has not yet undergone peer review, with the company planning to present more comprehensive results at a scientific conference later in the year.
Despite these gaps, the trial, encompassing nearly 18,000 adults with a history of cardiovascular disease tracked over up to five years, has strengthened the notion that obesity drugs can yield prolonged health benefits beyond weight loss. This challenges the perception of such drugs as mere aesthetic aids with limited impact on underlying health, potentially influencing insurers to reconsider coverage policies.
The trial’s focus on adults with prior cardiovascular issues may make it more challenging for insurers to deny coverage for non-diabetic patients. Craig Garthwaite, a health economist at Northwestern University’s Kellogg School of Management, anticipates increased difficulty in arguing against these drugs as part of essential health benefits, especially given their demonstrated cardiovascular advantages.
Medicare’s lack of coverage for weight loss medications, coupled with some employer insurance plans refusing to pay for them due to perceived non-essential medical benefits, creates a barrier for many individuals, especially considering the high list price of Wegovy at $1,349 per month.
The potential to reduce the risk of heart attacks or strokes not only holds the promise of alleviating suffering and medical complications for numerous Americans but also carries economic advantages. Dr. Garthwaite notes that this could restore productivity losses associated with heart disease and diminish spending on less effective obesity treatments.
However, he acknowledges that the benefits for patients’ heart health may take time to translate into direct cost savings for insurers. The competition between products from the new class of obesity treatment, particularly if they demonstrate similar cardiovascular benefits, could eventually lead to price reductions.
Wegovy is currently approved for chronic weight management in the United States. Novo Nordisk has expressed intentions to seek regulatory approval in the United States and Europe for additional medical indications, though specific indications were not disclosed.
Notably, another version of the same drug produced by Novo Nordisk, Ozempic, is approved for lowering blood sugar levels in adults with type 2 diabetes. A smaller trial indicated that Ozempic also reduced the risk of heart complications in diabetes patients.
Martin Holst Lange, the executive vice president for development at the company, expressed optimism about Wegovy’s potential to reshape the perception and treatment of obesity based on the latest trial results.
While the precise mechanism through which the drug reduces the risk of heart complications remains unclear, scientists suggest that the new class of obesity drugs may have direct effects on blood vessels and the heart. Studies on animals have indicated improved survival rates during heart attacks with the use of these drugs. Dr. Daniel Drucker, a senior scientist at the Lunenfeld Tanenbaum Research Institute in Toronto, noted that these drugs might also indirectly lower the risk of heart disease by addressing factors such as body weight, blood pressure, or inflammation.
Dr. Drucker emphasized that the trial’s additional details could help researchers understand the relationship between weight loss and cardiovascular benefits, shedding light on how Wegovy improves heart health. He sees this as the beginning of a new era in enhancing the health of individuals with obesity.
Despite these positive aspects, the trial will also provide insights into potential side effects, considering its scale and duration. Some patients have reported adverse effects like nausea, diarrhea, vomiting, constipation, and stomach pain, leading to discontinuation of the medication in some cases.
Experts anticipate that the trial’s outcomes could challenge the traditional approach to obesity, which often places the responsibility on individuals to manage their weight. Dr. Ania Jastreboff, an endocrinologist and obesity-medicine specialist at Yale University, stressed that obesity should be treated as a medical condition rather than a personal choice. The latest results underscore the importance of addressing obesity like any other disease, she emphasized
Credited Source: NYTIMES